
Helping the Healing
Mitochondria are the powerhouse of cells, and Kyle Quinn, assistant professor of biomedical engineering at the University of Arkansas, wants to use microscopy to understand how their function can change with age.
“Aging affects us all in different ways,” he says. “And, consequently, the function of our cells can change in different ways.”
Quinn focuses on monitoring cell metabolism over time, using a microscopy technique that detects the natural fluorescence of molecules in our mitochondria. This allows him to investigate the role mitochondria play in age-related diseases like Alzheimer’s, cardiovascular disorders, cancer, diabetes and obesity without perturbing or damaging our cells.
One of the applications of Quinn’s research is on non-healing wounds, which disproportionately affect people in Arkansas. The microscopy techniques he utilizes have been applied to cancer research for decades, but very little work has centered on its use in wound healing. So, he’s set out to change that.

A Complicated – and Expensive - Problem
Skin wounds, particularly for those who are elderly or diabetic, can quickly turn into a very challenging and expensive problem. Tens of billions of dollars are spent on wound care each year, but the medical profession lacks the necessary tools to diagnose and treat non-healing wounds. This is, in part, due to the scientific community’s incomplete understanding about why wounds don’t heal. They’re typically found on the lower extremities – often as diabetic foot ulcers or venous stasis ulcers – but can also occur in other locations when blood flow is restricted due to prolonged pressure on the skin.
“There aren’t a lot of ways to evaluate why wounds aren’t healing, which makes it difficult to treat,” Quinn says. “These are ticking time bombs in some ways, because if an infection takes hold, that’s when things get complicated. Amputation or death are common consequences, and the mortality rate for someone with a diabetic foot ulcer is higher than most cancers.”
Quinn started researching wound healing because he saw it as an underdeveloped area in biomedical engineering that wasn’t getting much attention. During his postdoctoral research at Tufts University, he utilized the natural fluorescence of mitochondria to monitor stem cell differentiation and the development of engineered tissues. He realized some of these techniques could be transferred to monitor skin wound healing and wrote a grant proposal that resulted in a National Institutes of Health Pathway to Independence Award, which took him from his postdoctoral work to a full-time faculty position at the U of A.
 Quinn received his Bachelor of Science from the University of Wisconsin-Madison and
a doctorate in bioengineering from the University of Pennsylvania.
Quinn received his Bachelor of Science from the University of Wisconsin-Madison and
a doctorate in bioengineering from the University of Pennsylvania.
“What appealed to me about the U of A was that it was a small department at the time, and everyone seemed very collegial and collaborative,” he says. “I could tell there were potential opportunities to collaborate and explore other applications for our fluorescence imaging techniques.”
Mapping Metabolism
In order to tackle the problem of non-healing wounds, it’s important to understand how the body normally handles the healing process.
Quinn explains that skin wound healing is a coordinated dance with many different cell types. Inflammatory cells come in to clean up the wound, and then additional cells arrive to deposit collagen and reform the mechanical scaffold that holds the skin together. The cells that make up blood vessels grow into the wound and provide oxygen and nutrients to the area. And then, when the environment is right, keratinocytes “crawl over” and form the protective barrier that is needed. Quinn likens the process to renovating a house: inflammatory cells are the exterminators that kill the unwanted pests, the cells depositing collagen are like the carpenters who come in to work, the endothelial cells are the plumbers of vasculature, and the keratinocytes serve as the roofers.
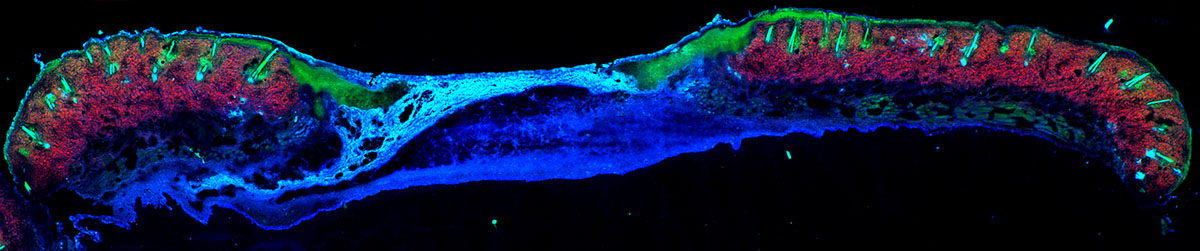
In Quinn’s lab, they use advanced microscopy technology to monitor the healing process and are establishing biomarkers to assess what the wounds are doing and what they need to heal. He explains that imaging techniques like MRI and CT scans don’t have great resolution, making it impossible to visualize individual cells, so he and his team instead have turned to multiphoton microscopy, a powerful imaging system capable of viewing biological tissue in three dimensions at the cellular level. The system allows him to generate 3D maps of wound metabolism based on the natural fluorescence of mitochondria.
 Multiphoton microscopy can enable non-invasive imaging in a clinical setting due
to several advantages compared to other microscopy techniques.
Multiphoton microscopy can enable non-invasive imaging in a clinical setting due
to several advantages compared to other microscopy techniques.
“When someone comments that your skin is glowing, it actually does glow,” Quinn says. “Our cells have a couple of naturally fluorescent molecules in them – much like what you would see when turning on a black light. They are primarily localized in mitochondria and are useful in understanding cell metabolism, which is the process through which our cells turn food into energy.”
When cells break down glucose and convert it into energy, they use a network of enzymatic reactions that produce adenosine triphosphate, more commonly referred to as ATP. Quinn explains that there are molecules that assist enzymes and connect the breakdown of sugar to the production of ATP, and they carry and release electrons. Whether they’re carrying an electron or not is something that can be detected. One molecule is fluorescent when it’s carrying electrons and one is fluorescent when it’s not carrying electrons.
These molecules are related to metabolism and can provide a sense for whether the cells are breaking down sugar to produce ATP or for other reasons. A ratio of these two fluorescence molecules can give researchers an idea about what the cell is doing – either growing and dividing or starting to “crawl” across the wound.
“At the edge of a wound, our cells metabolically act like a tumor. They pick up on the fact that there’s a lot of inflammation from the wound, and they’re going to grow and divide like an aggressive tumor,” Quinn said. “As the wound inflammation subsides, they’ll stop proliferating at the edge of the wound and crawl between the scab and wound bed and reform the outer layer of skin.”
For many older adults, the cells don’t grow and divide quickly enough at the beginning of the healing process. And with many diabetic patients, the cells remain at the wound edge and keep growing quickly but don’t crawl into the wound. Quinn’s imaging approach can detect these different responses between age-related delays and diabetic-related delays.
“If we can identify the cause of impaired healing on a case-by-case basis, we can learn to treat these wounds better,” he said.
A Rising Star in Research
Since Quinn joined the U of A in 2015, he has accumulated a multitude of research funding to support his work. Three of these awards are particularly notable.
In addition to these grants, Quinn received nearly $750,000 from the NIH in 2015, a $1.7 million NIH grant in 2017, $140,000 from the Department of Defense in 2018 to study the role that spinal cord injuries play in skin wound healing, the Rising Star Faculty Award from the university’s College of Engineering in 2019, and another NIH grant of nearly $400,000 for tools to assess mitochondrial diseases in 2019.
In 2019, Quinn earned $500,000 to support his research and teaching through the NSF’s Faculty Early Career Development program, known as a CAREER award. It’s considered one of the most prestigious awards in support of early-career faculty. One of the areas funded by the grant will be outreach activities in Arkansas, including summer camps offered by the Department of Biomedical Engineering that are designed to expose K-12 students to science – particularly from groups that are underrepresented in engineering.
Then, in 2021, he received a four-year, $1.6 million grant from the National Institutes of Health to develop non-invasive, real-time “optical biopsies” of chronic skin wounds. He is combining multiphoton microscopy and “deep learning,” an artificial intelligence-based approach to analysis, with the goal of training a computer algorithm to delineate wound regions accurately and quickly.
That same year, the NIH announced a $10.8 million grant to establish a center of biomedical research excellence that will enable an interdisciplinary team of researchers at the U of A and the University of Arkansas for Medical Sciences to address the role of cell and tissue metabolism in rare and common diseases such as cancer, diabetes and obesity. Quinn serves as director of the Arkansas Integrative Metabolic Research Center, which supports three research cores focused on providing complementary, state-of-the-art research tools to aid researchers in studying cell and tissue metabolism.
Because of these grants, Quinn can also fund the work of graduate students in his lab, who contribute to the research.
“I really enjoy training the next generation of scientists,” he said. “It’s one of the most rewarding aspects of the job. I am here because of the supportive and collaborative environment, and it has motivated me to help grow biomedical research on campus through our new center. We’ve got a good group of people who enjoy interacting with each other, which can be rare in academia.”

Alan Woessner, originally from the Northeast Arkansas town of Manila, came to the U of A as an undergraduate and has continued to work in Quinn’s lab since. He earned his doctorate from the university in 2022 and is the imaging and spectroscopy core manager for the Arkansas Integrative Metabolic Research Center.
“I like being on the cutting edge of this research,” he said. “Kyle is a good mentor. He pushes you to learn new things but also ensures you understand the bigger picture of what you’re doing and the primary goal. He keeps us grounded.”
Quinn notes that there can be other medical or scientific applications for the work he is doing, including aiding in the evaluation of new wound care products in the future. So, what started as an endeavor into an untapped area could bring additional breakthroughs in technology and will almost certainly guarantee that the scientific spotlight continues to shine on Quinn in Fayetteville.
“I feel like I can make an impact here,” he says.